Nous sommes fiers de nos découvertes
Avec >50 professeurs, collaborateurs universitaires, partenaires industriels et étudiants inscrits au programme FONCER-SFC, nous accumulons rapidement des résultats de recherche très prometteurs susceptibles de faire progresser la science en flux continu. Pour vous faciliter la tâche, la diffusion de nos travaux se retrouve dans cinq (5) catégories distinctes : les publications, les brevets, les présentations orales ou par affiches, les séminaires étudiants et les thèses ou mémoires.
NB : Les articles PDF ci-dessous sont directement liés aux fichiers hébergés sur les sites web de leur éditeur respectif. Si vous ou votre université souscrivez à un abonnement à ces revues électroniques, vous pouvez les télécharger simplement en cliquant sur le bouton vert. Sinon, vous pouvez communiquer avec les auteurs pour de plus amples renseignements.
- AUDUBERT, C.; LEBEL, H.
- Mild Esterification of Carboxylic Acids via Continuous Flow Diazotization of Amines
- Org. Lett. 2017, 19, 4407-4410.
A new continuous flow protocol for the diazotization of methylamine with 1,3-propanedinitrite in THF is reported. The synthesis of methyl esters was achieved in high yields from a variety of carboxylic acids in 20 min at 90 °C. Additionally, this protocol was extended to other aryl and alkyl amines, namely secondary amines, to produce various substituted esters in high yield using 2-MeTHF as a solvent. The reaction conditions were compatible with many functional groups, namely nitrogen-containing heterocycles, alkynes, alkenes, alcohols, and phenols. Mechanistic investigations reveal that the reaction appears to proceed through a transient diazonium species rather than a diazo intermediate.
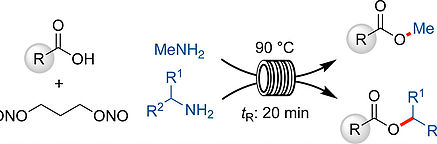
Lire l'article PDF
- AUDUBERT, C.; GAMBOA MARIN, O. J.; LEBEL, H.
- Batch and Continuous-Flow One-Pot Processes using Amine Diazotization to Produce Silylated Diazo Reagents
- Angew. Chem. Int. Ed. 2017, 56, 6294-6297.
A novel synthesis of trimethylsilyldiazomethane (TMSCHN2) by diazotization of trimethylsilylmethylamine (TMSCH2NH2) is reported using batch and continuous flow synthesis. The latter affords a daily production of 275 g (2.4 mol) of TMSCHN2. Other silylated methylamines were also successfully reacted under the developed reaction conditions to furnish various silicon-bearing diazomethane reagents. The applicability of the process is highlighted by disclosure of batch and continuous flow one-pot esterification and 1,3-dipolar cycloaddition processes. Furthermore, the high-yielding esterification of carboxylic acids with silylated and substituted methylamines in continuous flow is disclosed. Finally, work-up and purification procedures are reported for the preparation of a 2-MeTHF solution of TMSCHN2, which can be used in rhodium-catalyzed methylenation and homologation reactions.
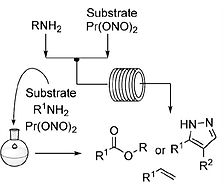
Lire l'article PDF
- GAREAU, D.; DESROSIERS, A.; VALLÉE-BÉLISLE, A.
- Programmable Quantitative DNA Nanothermometers
- Nano Lett. 2016, 16, 3976-3981.
Developing molecules, switches, probes or nanomaterials that are able to respond to specific temperature changes should prove of utility for several applications in nanotechnology. Here, we describe bioinspired strategies to design DNA thermoswitches with programmable linear response ranges that can provide either a precise ultrasensitive response over a desired, small temperature interval (±0.05 °C) or an extended linear response over a wide temperature range (e.g., from 25 to 90 °C). Using structural modifications or inexpensive DNA stabilizers, we show that we can tune the transition midpoints of DNA thermometers from 30 to 85 °C. Using multimeric switch architectures, we are able to create ultrasensitive thermometers that display large quantitative fluorescence gains within small temperature variation (e.g., > 700% over 10 °C). Using a combination of thermoswitches of different stabilities or a mix of stabilizers of various strengths, we can create extended thermometers that respond linearly up to 50 °C in temperature range. Here, we demonstrate the reversibility, robustness, and efficiency of these programmable DNA thermometers by monitoring temperature change inside individual wells during polymerase chain reactions. We discuss the potential applications of these programmable DNA thermoswitches in various nanotechnology fields including cell imaging, nanofluidics, nanomedecine, nanoelectronics, nanomaterial, and synthetic biology.
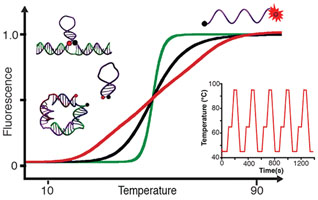
Lire l'article PDF
- LÉVESQUE, É.; LAPORTE, S. T.; CHARETTE, A. B.
- Continuous Flow Synthesis and Purification of Aryldiazomethanes via Hydrazone Fragmentation
- Angew. Chem. Int. Ed. 2017, 56, 837-841.
Electron-rich diazo compounds, such as aryldiazomethanes, are powerful reagents for the synthesis of complex structures, but the risks associated with their toxicity and instability often limit their use. Flow chemistry techniques make these issues avoidable, as the hazardous intermediate can be used as it is produced, avoiding accumulation and handling. Unfortunately, the produced stream is often contaminated with other reagents and by-products, making it incompatible with many applications, especially in catalysis. Herein is reported a metal-free continuous flow method for the production of aryldiazomethane solutions in a non-coordinating solvent from easily prepared, bench-stable sulfonylhydrazones. All by-products are removed by an in-line aqueous wash, leaving a clean, base-free diazo stream. Three successful sensitive metal-catalyzed transformations demonstrated the value of the method.
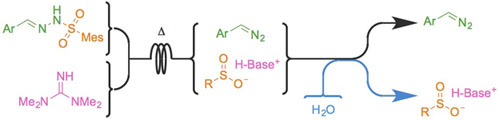
Lire l'article PDF
- GAREAU, D.; DESROSIERS, A.; VALLÉE-BÉLISLE, A.
- Programmable Quantitative DNA Nanothermometers
- Nano Lett. 2016, 16, 3976-3981.
Developing molecules, switches, probes or nanomaterials that are able to respond to specific temperature changes should prove of utility for several applications in nanotechnology. Here, we describe bioinspired strategies to design DNA thermoswitches with programmable linear response ranges that can provide either a precise ultrasensitive response over a desired, small temperature interval (±0.05 °C) or an extended linear response over a wide temperature range (e.g., from 25 to 90 °C). Using structural modifications or inexpensive DNA stabilizers, we show that we can tune the transition midpoints of DNA thermometers from 30 to 85 °C. Using multimeric switch architectures, we are able to create ultrasensitive thermometers that display large quantitative fluorescence gains within small temperature variation (e.g., > 700% over 10 °C). Using a combination of thermoswitches of different stabilities or a mix of stabilizers of various strengths, we can create extended thermometers that respond linearly up to 50 °C in temperature range. Here, we demonstrate the reversibility, robustness, and efficiency of these programmable DNA thermometers by monitoring temperature change inside individual wells during polymerase chain reactions. We discuss the potential applications of these programmable DNA thermoswitches in various nanotechnology fields including cell imaging, nanofluidics, nanomedecine, nanoelectronics, nanomaterial, and synthetic biology.

Lire l'article PDF
- HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Heteroleptic Cu-Based Sensitizers in Photoredox Catalysis
- Acc. Chem. Res. 2016, 49, 1557-1565.
Photochemistry is an important tool in organic synthesis that has largely been underdeveloped in comparison to thermal activation. Recent advances in technology have ushered in a new era in synthetic photochemistry. The emergence of photocatalysis, which exploits sensitizers for the absorption of visible light, has provided organic chemists with a new route to the generation of radical intermediates for synthesis. Of particular interest is the development of Cu-based complexes for photocatalysis, which possess variable photophysical properties and can display complementary reactivity with common photocatalysts based on heavier transition metals such as Ru or Ir. Heteroleptic Cu-based sensitizers incorporating the presence of both a bisphosphine and diamine ligand bound to the copper center are a promising class of photocatalysts. Their synthesis is a single step, often involving only precipitation for purification. In addition, it was shown that the sensitizers could be formed in situ in the reaction mixture, simplifying the experimental setup. The heteroleptic nature of the Cu-complexes also affords opportunities to fine-tune properties. For example, structurally rigidified bisphosphines reinforce geometries about the metal center to extend the excited state lifetime. Variation of the diamine ligand can influence the excited state oxidation/reduction potentials and optical absorbances. The heteroleptic complex Cu(XantPhos)(neo)BF4 has demonstrated utility in the synthesis of helical polyaromatic carbocycles. The synthesis of [5]helicene, a relatively simple member of the helicene family, was improved from the existing UV-light mediated method by eliminating the formation of unwanted byproducts. In addition, the Cu-based sensitizers also promoted the formation of novel pyrene/helicene hybrids for materials science applications. The synthetic methods that were developed were augmented when combined with continuous flow technology. The irradiation of reaction mixtures as they are pumped through small diameter tubing provides a more homogeneous and increased photon flux compared with irradiation in round-bottom flasks or other batch reactors. The value of continuous flow methods is also evident when examining UV-light photochemistry, where the simple and safe experimental set-ups allow for further exploration of high energy light for synthetic purposes. The synthesis of functionalized complex carbazoles was also studied using both a visible light method exploiting a heteroleptic copper-based sensitizer and a UVlight mediated method. It was demonstrated that both the photocatalysis methods and UV light photochemistries were rendered more user-friendly, safe, and reproducible when using continuous flow methods. Interestingly, the two photochemical methods often afford contrasting selectivities as a result of their inherently different mechanisms. It can be expected that the complementarity of the various photochemical methods will be an asset to synthetic chemists as the field continues to evolve.
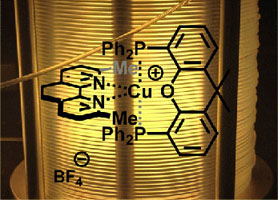
Lire l'article PDF
- DE LÉSÉLEUC, M.; GODIN, É.; PARISIEN-COLLETTE, S.; LÉVESQUE, A.; COLLINS, S. K.
- Catalytic Macrocyclization Strategies Using Continuous Flow: Formal Total Synthesis of Ivorenolide A
- J. Org. Chem. 2016, 81, 6750-6756.
A formal total synthesis of ivorenolide A has been accomplished employing a Z-selective olefin cross metathesis and a macrocyclic Glaser−Hay coupling as key steps. The macrocyclization protocol employed a phase separation/continuous flow manifold whose advantages include catalysis, fast reaction times, high concentrations, and facile scale-up.
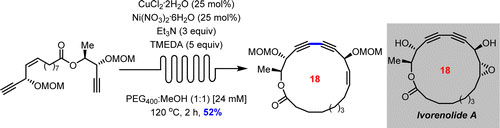
Lire l'article PDF
- PARISIEN-COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Photochemical Synthesis of Carbazoles Using an [Fe(phen)3](NTf2)2/O2 Catalyst System: Catalysis toward Sustainability
- Org. Lett. 2016, 18, 4994-4997.
An increasingly sustainable photochemical synthesis of carbazoles was developed using a catalytic system of Fe(phen)3(NTf2)2/O2 under continuous flow conditions and was demonstrated on gramscale using a numbering-up strategy. Photocyclization of triaryl and diarylamines into the corresponding carbazoles occurs in general in higher yields than with previously developed photocatalysts.
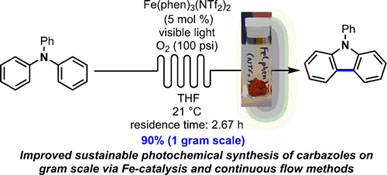
Lire l'article PDF
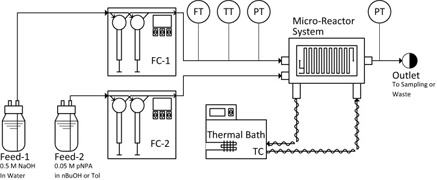
- MIELKE, E.; ROBERGE, D. M.; MACCHI, A.
- Microreactor Mixing-Unit Design for Fast Liquid–Liquid Reactions
- J. Flow Chem. 2016, 6, 279-287.
Based on previous work studying complex microreactors, it was desired to further improve the mixing efficiency by varying the mixing unit design for fast liquid—liquid reactions. Different flow regimes were studied, including slug flow, parallel flow, and drop flow. The two-phase hydrolysis of 4-nitrophenyl acetate in sodium hydroxide solution was used to evaluate the overall volumetric mass transfer coefficients (Korga) as a function of the average rate of energy dissipation (ε) for each microreactor design and all flow regimes. The liquid—liquid systems investigated used n-butanol or toluene as the organic phase solvent and a 0.5-M NaOH aqueous solution. The use of surfactant was also investigated with the toluene—water system. All microreactor geometry designs were based on contraction—expansion repeating units with asymmetric obstacles to aid the breakup of slugs and desynchronize the recombination of split streams. The investigated designs were chosen to avoid the formation of the parallel flow regime, contrary to curvature-based mixing-unit designs. The microreactor design can then be optimized to reduce the ε required to reach drop flow, since Korga has been found to be constant at equal ε for a given solvent system in this flow regime, regardless of the reactor selection. Additionally, the "3/7th" scaleup rule was applied and confirmed with the LL-Triangle mixer. It was found that, for low interfacial-tension systems (i.e., n-butanol—water), the onset of drop flow occurred at a lower ε for the LL-Triangle mixer when compared with the Sickle or LL-Rhombus mixers.
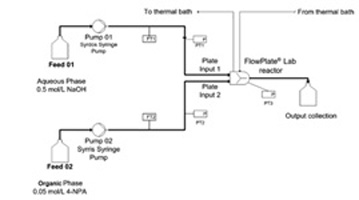
Lire l'article PDF
- PLOUFFE, P.; ROBERGE, D. M.; MACCHI, A.
- Liquid-Liquid Flow Regimes and Mass Transfer in Various Micro-Reactors
- Chem. Eng. J. 2016, 300, 9-19.
The flow regimes and mass transfer rates in five complex micro-reactors with different mixing mechanisms were investigated using the two-phase alkaline hydrolysis of 4-nitrophenyl acetate. n-Butanol and toluene were used as organic solvents. Using n-butanol in curvature-based micro-mixers, the flow regime evolved from slug to parallel to drop/dispersed flow with increasing flow rates. In obstacle-based micro-mixers, no parallel flow was observed. Using toluene, no parallel flow was observed for all reactors. The conversion of 4-nitrophenyl acetate was found to be strongly dependent on the flow regime. In slug and parallel flow, the conversion generally decreased with an increase in flow rate whereas it typically increased in drop flow and was constant or slightly decreased in dispersed flow. The different micro-mixers were compared using the overall volumetric mass transfer coefficient, Korga, which was primarily a function of the rate of energy dissipation within the dispersed flow regime. The geometry itself impacts the resulting flow regime and rate of energy dissipation at a given flow rate. The micro-reactors were then compared using modified Damköhler's numbers. Curvature-based reactors were found to be inadequate for liquid–liquid reactions under the studied conditions, as they favor parallel flow patterns and yield relatively low interphase mass transfer rates.
Lire l'article PDF
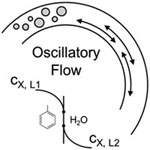
- MONGEON, S. S.; ROBERGE, D. M.; BITTEL, M.; ELSNER, P.; MACCHI, A.
- Liquid–Liquid Mass Transfer in an Oscillatory-Flow Mesoscale Coil Reactor without Baffles
- Org. Process Res. Dev. 2016, 20, 733-741.
Interphase mass transfer rates of an immiscible liquid−liquid system are investigated in an oscillatory-flow coil reactor without baffles. A baffle-less system is chosen since much research has been completed in baffled tubes with little performance comparison to baffle-less tubes, which offer lower operating and capital costs. In our experiments, interphase mass transfer rates are evaluated via the two-phase alkaline hydrolysis of 4-nitrophenyl acetate (4-NPA) in a toluene−water biphasic system. The mass transfer rate increased 7-fold at the maximum tested oscillation of 5000 oscillatory-flow Reynolds number. The best application for the oscillatory-flow coil reactor is determined using a comparison to other liquid−liquid flow platforms in the presented toolbox approach. The oscillatory-flow coil reactor becomes a clear complement to a plate microreactor for gaining volume.
Lire l'article PDF
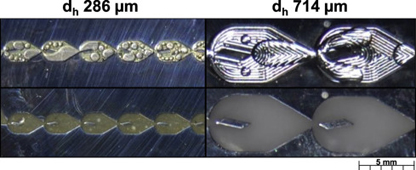
- PLOUFFE, P.; ROBERGE, D. M.; BITTEL, M.;SIEBER, J.; MACCHI, A.
- On the Scale-Up of Micro-Reactors for Liquid-Liquid Reactions
- Chem. Eng. Sci. 2016, 143, 216-225.
The scale-up of a micro-reactor with a mixer designed for liquid–liquid reactions is investigated using a 3/7th approach that scales the hydraulic diameter at increased flow rates and keeps constant the average rate of energy dissipation. Smaller (dh 283 μm) and larger-scale (dh 714 μm) mixers are compared using single phase pressure drop measurements and a liquid–liquid reactive extraction. The single phase tests demonstrate that the energy dissipation rate of the larger scale mixer is comparable to the smaller scale mixer at flow rates ~8.5 times greater. The overall volumetric mass transfer coefficients of the reactive extraction, Kca, of both scale mixers were also similar at equal energy dissipation rate in the drop flow regime. Finally, the upper limit of the sizing approach is discussed and compared with commercially available static mixers.
Lire l'article PDF
- LEBEL, H.; PIRAS, H.; BORDUY, M.
- Iron-Catalyzed Amination of Sulfides and Sulfoxides with Azides in Photochemical Continuous Flow Synthesis
- ACS Catal. 2016, 6, 1109–1112.
A photochemical (UVA) continuous flow process for the amination of thioethers and sulfoxides was performed with trichloroethoxysulfonyl azide in the presence of catalytic iron(III) acetylacetonate. Aromatic and aliphatic sulfilimines and sulfoximines were produced in high yields and short reaction times. The reaction with chiral sulfoxides was stereospecific, producing enantioenriched sulfoximines in excellent yields.

Lire l'article PDF
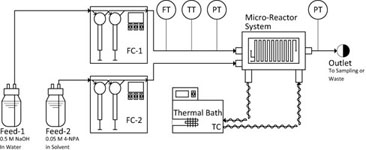
- PLOUFFE, P.; ROBERGE, D.M.; SIEBER, J.; BITTEL, M.; MACCHI, A.
- Liquid-Liquid Mass Transfer in a Serpentine Micro-Reactor Using Various Solvents
- Chem. Eng. J. 2016, 285, 605–615.
The flow regimes are identified and mass transfer rates measured in a serpentine micro-reactor with a hydraulic diameter of 0.7 mm using the two-phase alkaline hydrolysis of 4-nitrophenyl acetate between flow rates of 0.8 and 32 mL/min. Four different organic solvents (n-butanol, n-hexanol, MTBE and toluene) were used in order to investigate the effect of phase physical properties. The continuous phase overall volumetric mass transfer coefficient, Kca, was greatest in the drop flow regime and for organic solvents with lower interfacial tension resulting in larger interfacial area, a. In the parallel flow regime, Kca values increased linearly with the continuous phase capillary number. In the drop flow regime, two correlations for the prediction of the drop size were developed with fitted parameters falling within the expected values for turbulent break-up of drops in conventional static mixers.
Lire l'article PDF
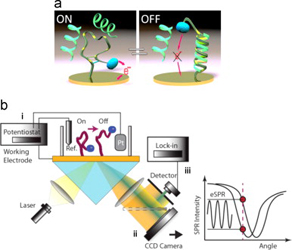
- DALLAIRE, A. M.; PATSKOVSKY, S.; VALLÉE-BÉLISLE, A.; MEUNIER, M.
- Electrochemical Plasmonic Sensing System for Highly Selective Multiplexed Detection of Biomolecules Based on Redox Nanoswitches
- Biosens Bioelectron. 2015, 71, 75-81.
In this paper,we present the development of a nanoswitch-based electrochemical surface plasmon resonance (eSPR) transducer for the multiplexed and selective detection of DNA and other biomolecules directly in complex media. To do so, we designed an experimental set-up for the synchronized measurements of electrochemical and electro-plasmonic responses to the activation of multiple electro- chemically labeled structure-switching biosensors. As a proof of principle, we adapted this strategy for the detection of DNA sequences that are diagnostic of two pathogens (drug-resistant tuberculosis and Escherichia coli) by using methylene blue-labeled structure-switching DNA stem-loop. The experimental sensitivity of the switch-based eSPR sensor is estimated at 5 nM and target detection is achieved within minutes. Each sensor is reusable several times with a simple 8M urea washing procedure. We then demonstrated the selectivity and multiplexed ability of these switch-based eSPR by simultaneously detecting two different DNA sequences. We discuss the advantages of the proposed eSPR approach for the development of highly selective sensor devices for the rapid and reliable detection o fmultiple molecular markers in complex samples.
Lire l'article PDF
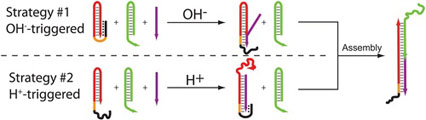
- IDILI, A.; PORCHETTA, A.; PATSKOVSKY, S.; VALLÉE-BÉLISLE, A.; RICCI, F.
- Controlling Hybridization Chain Reaction with pH
- Nano Lett. 2015, 15, 5539–5544.
By taking inspiration from nature, where selforganization of biomolecular species into complex systems is finely controlled through different stimuli, we propose here a rational approach by which the assembly and disassembly of DNA-based concatemers can be controlled through pH changes. To do so we used the hybridization chain reaction (HCR), a process that, upon the addition of an initiator strand, allows to create DNA-based concatemers in a controlled fashion. We re-engineered the functional units of HCR through the addition of pH-dependent clamp-like triplex-forming domains that can either inhibit or activate the polymerization reaction at different pHs. This allows to finely regulate the HCR-induced assembly and disassembly of DNA concatemers at either basic or acidic pHs in a reversible way. The strategies we present here appear particularly promising as novel tools to achieve better spatiotemporal control of self-assembly processes of DNA-based nanostructures.
Lire l'article PDF
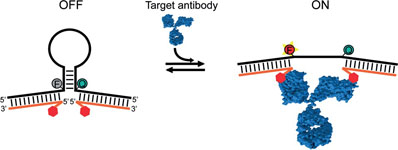
- RANALLO, S.; ROSETTI, M.; PLAXCO, K. W.; VALLÉE-BÉLISLE, A.; RICCI, F.
- A Modular, DNA-Based Beacon for Single-Step Fluorescence Detection of Antibodies and Other Proteins
- Angew. Chem. Int. Ed. 2015, 54, 13214–13218.
A versatile platform for the one-step fluorescence detection of both monovalent and multivalent proteins has been developed. This system is based on a conformationswitching stem–loop DNA scaffold that presents a smallmolecule, polypeptide, or nucleic-acid recognition element on each of its two stem strands. The steric strain associated with the binding of one (multivalent) or two (monovalent) target molecules to these elements opens the stem, enhancing the emission of an attached fluorophore/quencher pair. The sensors respond rapidly (>10 min) and selectively, enabling the facile detection of specific proteins even in complex samples, such as blood serum. The versatility of the platform was demonstrated by detecting five bivalent proteins (four antibodies and the chemokine platelet-derived growth factor) and two monovalent proteins (a Fab fragment and the transcription factor TBP) with low nanomolar detection limits and no detectable cross-reactivity.
Lire l'article PDF
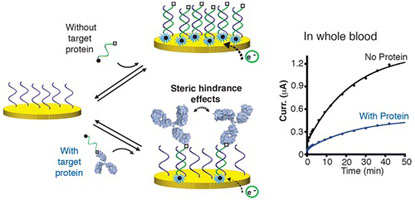
- MAHSHID, S.; CAMIRÉ, S.; RICCI, A.; VALLÉE-BÉLISLE, A.
- A Highly Selective Electrochemical DNA-Based Sensor that Employs Steric Hindrance Effects to Detect Proteins Directly in Whole Blood
- J. Am. Chem. Soc. 2015, 137, 15596–15599.
Here we describe a highly selective DNA based electrochemical sensor that utilizes steric hindrance effects to signal the presence of large macromolecules in a single-step procedure. We first show that a large macromolecule, such as a protein, when bound to a signaling DNA strand generates steric hindrance effects, which limits the ability of this DNA to hybridize to a surface-attached complementary strand. We demonstrate that the efficiency of hybridization of this signaling DNA is inversely correlated with the size of the molecule attached to it, following a semilogarithmic relationship. Using this steric hindrance hybridization assay in an electrochemical format (eSHHA), we demonstrate the multiplexed, quantitative, one-step detection of various macromolecules in the low nanomolar range, in >10 min directly in whole blood. We discuss the potential applications of this novel signaling mechanism in the field of point-of-care diagnostic sensors.
Lire l'article PDF
- LOFTI, S.; BOFFITO, D. C.; PATIENCE, G. S.
- Gas-Phase Partial Oxidation of Lignin to Carboxylic Acids over Vanadium Pyrophosphate and Aluminum–Vanadium–Molybdenum
- ChemSusChem 2015, 8, 3424-3432.
Lignin is a complex polymer that is a potential feedstock for aromatic compounds and carboxylic acids by cleaving the β-O-4 and 5-5' linkages. In this work, a syringe pump atomizes an alkaline solution of lignin into a catalytic fluidized bed operating above 600 K. The vanadium heterogeneous catalysts convert all the lignin into carboxylic acids (up to 25% selectivity), coke, carbon oxides, and hydrogen. Aluminum–vanadium–molybdenum mostly produced lactic acid (together with formic acid, acrylic acid, and maleic anhydride), whereas the vanadium pyrophosphate catalyst produced more maleic anhydride.
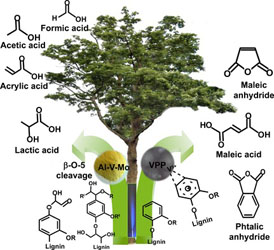
Lire l'article PDF
- DALIL, M.; CARNEVALI, D.; DUBOIS, J-L; PATIENCE, G. S.
- Transient Acrolein Selectivity and Carbon Deposition Study of Glycerol Dehydration over WO3/TiO2 Catalyst
- Chem. Eng. J. 2015, 270, 557–563.
Acrolein is one of the highest valued commodity chemicals for which glycerol is an attractive feedstock. Glycerol is derived from transesterification of vegetable oils and animal fats. WO3/TiO2 catalyst dehydrated glycerol to acrolein at a selectivity exceeding 73% after 6 h time-on-stream. However, after 1 h, the acrolein selectivity was only 55%. All the glycerol reacted at reaction temperature (280 °C) and the by-products were predominantly propanal, acetaldehyde, formic and acetic acids. The weight gain of carbon on the catalyst increased with time and after one hour, the mass fraction of carbon was 2.2%; after an additional five hours, it doubled. A first order kinetic model characterizes the rate at which oxygen reacts the carbon on the catalyst with an activation energy of 100 kJ mol-1 (R2 >0.997). Partial oxidation of coke, as a new strategy for catalyst regeneration, enhanced the selectivity toward acrolein from 10% to 25% in the first 15 min of glycerol injection. Glycerol conversion to coke was reduced from 34% to 6%.
Lire l'article PDF
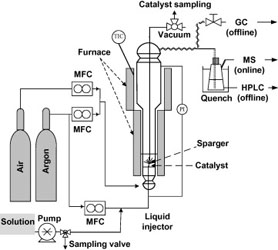
- BREAULT-TURCOT, J.; MASSON, J.-F.
- Microdialysis SPR: Diffusion-Gated Sensing in Blood
- Chem. Sci. 2015, 6, 4247-4254.
Chemical measurements are rarely performed in crude blood due to the poor performance of sensors and devices exposed to biofluids. In particular, biosensors have been severely limited for detection in whole blood due to surface fouling from proteins, the interaction of cells with the sensor surface and potential optical interference when considering optical methods of analysis. To solve this problem, a dialysis chamber was introduced to a surface plasmon resonance (SPR) biosensor to create a diffusion gate for large molecules. This dialysis chamber relies on the faster migration of small molecules through a microporous membrane towards a sensor, located at a specified distance from the membrane. Size filtering and diffusion through a microporous membrane restricted the access of blood cells and larger biomolecules to a sensing chamber, while smaller, faster diffusing biomolecules migrated preferentially to the sensor with limited interference from blood and serum. The affinity of a small peptide (DBG178) with anti-atherosclerotic activity and targeting type B scavenger receptor CD36 was successfully monitored at micromolar concentrations in human serum and blood without any pre-treatment of the sample. This concept could be generally applied to a variety of targets for biomolecular interaction monitoring and quantification directly in whole blood, and could find potential applications in biochemical assays, pharmacokinetic drug studies, disease treatment monitoring, implantable plasmonic sensors, and point-of-care diagnostics.
Lire l'article PDF
- BOFFITO, D. C.; BLANCO-MANRIQUE, G.; PATIENCE, G. S.
- One-Step Cracking/Transesterification of Vegetable Oil: Reaction-Regeneration Cycles in a Capillary Fluidized Bed
- Energy Convers. Manage. 2015, 103, 958–964.
CaO/Al2O3 catalytically transesterifies and cracks vegetable oils in the gas phase at temperatures beyond 400 °C. The products are biodiesel (fatty acid methyl esters) and hydrocarbons. Oil conversion was complete with excess methanol in the feed. The gas residence time was less than 1 s and the weight-hourly-space velocity in the capillary fluidized bed was on the order of 5 h-1 (0.12 mg min-1 oil feed rate with 1.6 g of catalyst). Periodic regeneration cycles with O2 converted the coke on the catalyst to CO and CO2. Regenerated catalyst is initially less selective towards biodiesel. Coke preserves the active sites that are selective for the conversion of the triglycerides into biodiesel. The biodiesel yield was highest (44%) dosing the catalyst with oil for 5 min then regenerating it for 1 min.
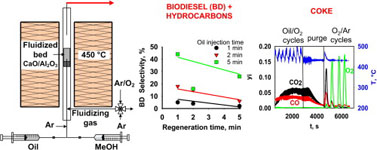
Lire l'article PDF
- TIEN SING YOUNG, R. V.; TABRIZIAN, M.
- Rapid, One-Step Fabrication and Loading of Nanoscale 1,2-Distearoyl-Sn-Glycero-3-Phosphocholine Liposomes in a Simple, Double Flow-Focusing Microfluidic Device
- Biomicrofluidics 2015, 9, 1-8.
Liposomes are currently well-established as biocompatible delivery vehicles for numerous compounds. However, conventional manufacturing tends to rely on time-consuming processes, costly equipment, unstable reaction parameters, and numerous pre- and post-processing steps. Herein, we demonstrate a microscope-slide-sized alternative: a double flow-focusing microfluidic geometry capable of sub-hour synthesis and controlled loading of tunable liposomes. Using phospholipid 1,2-distearoyl-sn-glycero-3-phosphocholine as the bilayer constituent, the effect of varying the dissolved lipid concentration and flow rate ratio on synthesized liposome diameters was investigated and the encapsulation of fluorescent hydrophobic drug model ergost-5,7,9(11),22-tetraen-3b-ol was performed to ascertain the potential of this device as a loading platform.
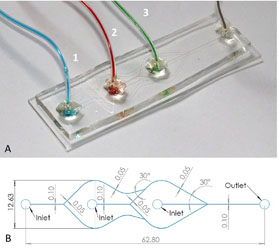
Lire l'article PDF
- BREAULT-TURCOT, J.; POIRIER-RICHARD, H.-P.; COUTURE, M.; PELECHACZ, D.; MASSON, J.-F.
- Single Chip SPR and Fluorescent ELISA Assay of Prostate Specific Antigen
- Lab Chip 2015, 15, 4433-4440.
A multi-channel system combining fluidics and micropatterned plasmonic materials with wavelength interrogation surface plasmon resonance (SPR) and fluorescence detection was integrated from the combination of a small and motorized fluorescence microscope mounted on a portable 4-channel SPR instrument. The SPR and fluorescent measurements were performed based on the same detection area in a multichannel fluidic, with a sensing scheme for prostate-specific antigen (PSA) consisting of a sandwich assay with a capture anti-PSA immobilized onto the SPR sensor and a detection anti-PSA modified with horseradish peroxidase (HRP). In this dual-detection instrument, fluorescence was measured from the solution side of the micropatterned gold film, while the interface between the glass prism and the gold film served to interrogate the SPR response. The SPR sensors were comprised of microhole arrays fabricated by photolithography to enhance the instrumental response for PSA detection by approximately a factor of 2 to 3 and they were coated with a self-assembled monolayer of a peptide (3-MPA-HHHDD-OH) to minimize nonspecific adsorption. PSA was successfully detected at clinical concentrations from 10 pM to 50 nM with this integrated system in a single assay lasting 12 minutes, almost centering on the desired range for PSA diagnostic tests (>4 ng mL−1 or >150 pM). The combination of two robust techniques in a single chip and instrument has led to a simple and effective assay that can be carried out on a small and portable instrument providing rapid biodetection of an important cancer biomarker with a dynamic range of nearly 4 orders of magnitude in the clinical range.
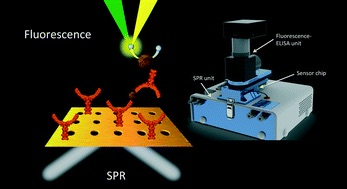
Lire l'article PDF
- BOFFITO, D. C.; GALLI, F.; MARTINEZ, P. R.; PIROLA, C.; BIANCHI, C. L.; PATIENCE, G. S.
- Transesterification of Triglycerides in a New Ultrasonic Assisted Mixing Device
- Chem. Eng. Trans. 2015, 43, 427-432.
We adapted a media mill to house an ultrasonic horn to transesterify triglycerides to biodiesel. This configuration combines enhanced mass transfer from the acoustic cavitation of the ultrasonic probe with nonconventional mixing from the media mill. It ensures oil and methanol circulates in the vicinity of the ultrasonic tip. We tested methanol, ethanol and isopropanol as alcohols and KOH as a catalyst to transesterify canola oil. The branched isopropanol lowers the biodiesel cloud point by 10 °C compared to biodiesel produced with methanol (fatty acid methyl ester). We withdrew samples at regular intervals and analysed them in a GC according to EN14103-2011. The ultrasonic-assisted mixing device that we designed converts most of the triglycerides with methanol within one minute of pulsed ultrasonic irradiation. The reaction with ethanol and isopropanol is faster than in classical batch reactors.

Lire l'article PDF
- HERNANDEZ-PEREZ, A.; CARON, A.; COLLINS, S. K.
- Photochemical Synthesis of Complex Carbazoles: Evaluation of Electronic Effects in Both UV- and Visible-Light Methods in Continuous Flow
- Chem. Eur. J. 2015, 21, 16673-16678.
An evaluation of both a visible-light- and UV-lightmediated synthesis of carbazoles from various triarylamines with differing electronic properties under continuous-flow conditions has been conducted. In general, triarylamines bearing electron-rich groups tend to produce higher yields than triarylamines possessing electron-withdrawing groups. The incorporation of nitrogen-based heterocycles, as well as halogen-containing arenes in carbazole skeletons, was well tolerated, and often synthetically useful complementarity was observed between the UV-light and visible-light (photoredox) methods.
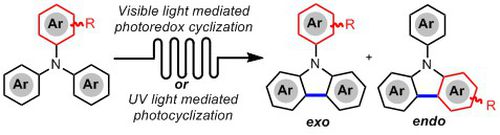
Lire l'article PDF
- BÉDARD, A.-C.; SANTANDREA, J.; COLLINS, S. K.
- Efficient Continuous-Flow Synthesis of Macrocyclic Triazoles
- J. Flow Chem. 2015, 5, 142-144.
The continuous-flow synthesis of a series of 11- to 26-membered macrocycles via copper-catalyzed azide–alkyne cycloaddition is reported. The approach employs homogeneous catalysis to promote formation of triazole-containing macrocycles in good to excellent yields (65–90%) at relatively high concentration (30–50 mM) using a phase separation strategy.
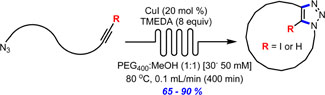
Lire l'article PDF
- CARON, A.; HERNANDEZ PEREZ, A. C.; COLLINS, S. K.
- Continuous Flow Reactor for UV-Light Mediated Synthesis
- Org. Process Res. Dev. 2014, 18, 1571-1574.
A continuous flow UV light reactor has been constructed using commercially available equipment, and its efficiency was demonstrated by performing a photocyclodehydrogenation reaction to prepare carbazole derivatives of the drug carprofen.
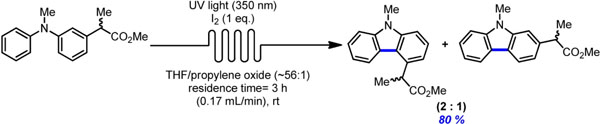
Lire l'article PDF
- BÉDARD, A.-C.; COLLINS, S. K.
- Advanced Strategies for Efficient Macrocyclic Cu(I)-Catalyzed Cycloaddition of Azides
- Org. Lett. 2014, 16, 5286-5289.
An advanced strategy for efficient macrocyclic Cu(I)-catalyzed cycloaddition is described. The key features include employing azide−iodoalkyne cycloadditions (CuAiAC), low catalyst loadings, relatively high concentrations (30 mM to 300 mM), and application to continuous flow. The remarkably efficient new tool affords a variety of macrocyclic skeletons having either different alkyl, aryl, or amino acid spacers in high yields (70-97%). The macrocyclic CuAiAC process affords macrocycles having an iodotriazole moiety that can be further functionalized using standard Pd-catalyzed cross-couplings.
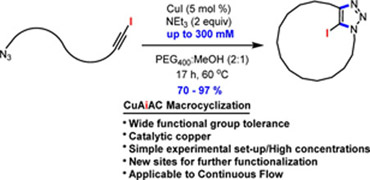
Lire l'article PDF
- PLOUFFE, P.; MACCHI, A.
- From Batch to Continuous Chemical Synthesis -- A Toolbox Approach
- Org. Process Res. Dev. 2014, 18, 1286-1294.
A continuous flow UV light reactor has been constructed using commercially available equipment, and its efficiency was demonstrated by performing a photocyclodehydrogenation reaction to prepare carbazole derivatives of the drug carprofen.
Lire l'article PDF
- CYR, P.; CHARETTE, A. B.
- Continuous-Flow Hydroxylation of Aryl Iodides Promoted by Copper Tubing
- Synlett 2014, 1409-1412.
A simple and ligand-free synthesis of phenols from the corresponding aryl iodides in a continuous-flow system is described. The reaction is complete in only 4 to 20 minutes when heated between 150 and 165 °C in a reactor consisting of a commercially available copper coil. An example of trapping of the phenoxide in situ is also shown.
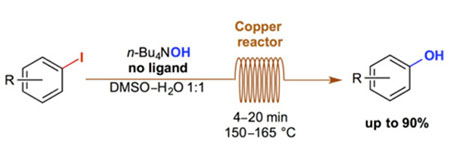
Lire l'article PDF
- ROBERGE, D.M.; PLOUFFE, P.; MACCHI, A.
- Fluid Mixing Structure, Reaction Unit, Reactor and Method Using the Same
- Demande de brevet soumise en décembre 2015. (No EP14196803.2)
- VALLÉE-BÉLISLE, A.
- Steric-Hindrance Hybridization Assays and Methods Associated Thereto
- Demande de brevet soumise en avril 2015. (No US 61/975, 231)
- BREAULT-TURCOT, J.; MASSON, J-F.
- Method and Device for Analysing a Sample of a Crude Biofluid
- Demande de brevet soumise en mars 2015. (No US 62/132,107)
- PARISIEN-COLLETTE, S.; COLLINS, S. K.
- Photochemical Synthesis of Carbazoles via Continuous Flow
- Flow Chemistry Congress, Cambridge, UK, 7-8 févr. 2017; Poster.
- SANTANDREA, J.; MINOZZI, C.; CRUCHÉ, C.; COLLINS, S. K.
- Photochemical Synthesis of Alkynyl Sulfides in Continuous Flow
- Flow Chemistry Congress, Cambridge, UK, 7-8 févr. 2017; Poster.
- GODIN, É.; COLLINS, S. K.
- Synthesis of Complex Macrocycles Usiing Phase Separation and Continuous Flow Strategies
- Flow Chemistry Congress, Cambridge, UK, 7-8 févr. 2017; Poster.
- MORIN, É.; COLLINS, S. K.
- Total Synthesis of Neomarchantin A via a Ring Closing Metathesis in Continuous Flow
- Flow Chemistry Congress, Cambridge, UK, 7-8 févr. 2017; Poster.
- CHARETTE, A. B.
- Development of New Synthetic Methods in Batch and in Flow
- 20th Sigma-Aldrich Symposium, Blankenberge, Belgium, 8-9 déc. 2016; Conférence sur invitation.
- CRIFAR, C.; LUBELL, W. D.
- Indole Synthesis by Flow Chemistry
- 19th Annual Chemistry & Biochemistry Graduate Research Conference, Montréal, QC, 18 nov. 2016; Oral.
- MORIN, É.; COLLINS, S. K.
- Total Synthesis of Neomarchantin A
- 19th Annual Chemistry & Biochemistry Graduate Research Conference, Montreal, QC, 18 nov. 2016; Oral.
- GODIN, É.; COLLINS, S. K.
- Total Synthesis of Ivorenolide A
- 19th Annual Chemistry & Biochemistry Graduate Research Conference, Montreal, QC, 18 nov. 2016; Oral.
- MINOZZI, C.; COLLINS, S. K.
- Copper-Based Catalysts in Photocatalysis
- 19th Annual Chemistry & Biochemistry Graduate Research Conference, Montreal, QC, 18 nov. 2016; Oral.
- SANTANDREA J.; COLLINS, S. K.
- Alkynyl Sulfide Synthesis via Photocatalysis
- 19th Annual Chemistry & Biochemistry Graduate Research Conference, Montreal, QC, 18 nov. 2016; Oral.
- RULLIÈRE, P.; PONS, A.; CHARETTE, A. B.
- Non-Stabilized Alkyldiazomethanes Continuous Flow Synthesis
- Flow Chemistry Congress, Miami, FL, 2-3 nov. 2016; Poster.
- KAIROUZ, V. K.; COLLINS, S. K.; CHARETTE, A. B.
- NSERC CREATE Program in Continuous Flow Science
- Flow Chemistry Congress, Miami, FL, 2-3 nov. 2016; Poster.
- CHARETTE, A. B.
- Novel Conditions for the Preparation of Diazo Reagents in Flow and their Applications
- Flow Chemistry Congress, Miami, FL, 2-3 nov. 2016; Conférence sur invitation.
- MACCHI, A.
- Development of Continuous Flow Micro-reactors for Fast Liquid and Liquid-liquid Reactions
- Flow Chemistry Congress, Miami, FL, 2-3 nov. 2016; Conférence sur invitation.
- COLLINS, S. K.
- Macrocyclization in Continuous Flow
- Flow Chemistry Congress, Miami, FL, 2-3 nov. 2016; Conférence sur invitation.
- MA, Z.; PELEGRIN, D. C.; BOFFITO, D. C.; PATIENCE, G. S.
- FeCralloy® Partially Oxidizes Methane to Syngas Selectively
- 66th Canadian Chemical Engineering Conference, Québec, QC, 16-19 oct. 2016; Oral.
- CARNEVALI, D.; PATIENCE, G. S.
- Glucose to 6-C Carboxylic Acids
- 66th Canadian Chemical Engineering Conference, Québec, QC, 16-19 oct. 2016; Oral.
- COLLINS, S. K.
- Continuous Flow Strategies in the Synthesis of Macrocycles and Heterocycles
- Vertex Canada, Laval, QC, 1 sept. 2016; Conférence sur invitation.
- COLLINS, S. K.
- Macrocycle Synthesis Using Continuous Flow
- 2nd International Symposium on Middle Molecular Strategy, Osaka, Japon, 2 juil. 2016; Conférence plénière.
- COLLINS, S. K.
- Photochemical Synthesis in Continuous Flow
- Group for Research on Automated Flow and Microreactor Synthesis, Osaka, Japon, 1 juil. 2016; Conférence plénière.
- COLLINS, S. K.
- Phase Separation for the Synthesis of Complex Macrocycles
- 99th Canadian Chemistry Conference and Exhibition, Halifax, QNS, 5-9 juin 2016; Oral.
- GODIN, É.; COLLINS, S. K.
- Total Synthesis of Ivorenolide A
- 7e conférence annuelle du CCVC, Montréal, QC, 2 juin 2016; Poster.
- MIELKE, E.; ROBERGE, D. M.; MACCHI, A.
- Micro-Reactor Mixing-Unit Design for Fast Liquid-Liquid Reactions
- Zing Continuous Flow Chemistry Conference, Albufeira, Portugal, 25-28 avr. 2016; Oral.
- COLLLINS, S. K.
- Photochemical Synthesis in Continuous Flow
- Zing Continuous Flow Chemistry Conference, Albufeira, Portugal, 25-28 avr. 2016; Conférence sur invitation.
- CHARETTE, A. B.
- New Cyclopropanation Methods in Batch and in Continuous Flow
- Journée de printemps, Organic Chemistry Division, French Chemical Society, Orsay, France, 22 mars 2016; Conférence sur invitation.
- GODIN, É.; COLLINS, S. K.
- Advanced Synthetic Strategies for Macrocyclization: Novel Catalysis, Concentration Control and Continuous Flow Strategies
- Delmar Chemicals Inc., LaSalle, QC, 19 févr. 2016; Conférence sur invitation.
- LÉVESQUE, É.; CHARETTE, A. B.
- On-demand Diazo Reagents : In-Flow Generation and Purification
- Delmar Chemicals Inc., LaSalle, QC, 19 févr. 2016; Conférence sur invitation.
- PIRAS, H.; LEBEL, H.
- Méthodes d'amination de thioéthers et de sulfoxydes à partir d'azotures sous activation photochimique en débit continu
- Delmar Chemicals Inc., LaSalle, QC, 19 févr. 2016; Conférence sur invitation.
- RULLIERE, P.; CYR, P.; CHARETTE, A. B.
- Difluorocyclopropanation of Alkenes and Alkynes in Continuous Flow
- 6th International Conference of The Flow Society, Cambridge, Royaume-Uni, 16-18 févr. 2016; Poster.
- KAIROUZ, V. K.; COLLINS, S. K.; CHARETTE, A. B.
- NSERC CREATE Program in Continuous Flow Science
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- COLLINS, S. K.
- Photochemical Transformations Using Alternative Sensitizers in Continuous Flow
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Conférence sur invitation.
- SANTANDREA, J.; BÉDARD, A.-C.; MINOZZI, C.; COLLINS, S. K.
- Advances in Thermal Photochemical Cu(I)-Catalysed Macrocyclic Sonogashira-Type Cross-Couplings
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- CARON, A.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- U.V. Light-Mediated Synthesis of Carbazoles Using Flow Chemistry
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- PARISIEN-COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Photoredox of Heterocycles in Continuous Flow
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- DE LÉSÉLEUC, M.; GODIN, É.; PARISIEN-COLLETTE, S.; COLLINS, S. K.
- Progress Toward the Total Synthesis of Ivorenolide A
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Oral.
- AUBÉ, A.; CHARBONNEAU, D.; PELLETIER, J.; MASSON, J.-F.
- Early Detection of Anti-Asparaginase to Significantly Increase Remission Rate in Acute Lymphoblastic Leukemia Therapy
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- LEBEL, H.; PIRAS, H.; BORDUY, M.
- Metal-Catalyzed Amination Processes in Continuous Flow Synthesis
- Pacifichem, Honolulu, HI, 15-20 déc., 2015; Poster.
- HAVARD, T.; SCHMITZER, A. R.; MASSON, J.-F.
- Molecularly Imprinted Polymeric Ionic Liquids Films for Plasmon Resonance Sensing of Energetic Materials in Aqueous Media
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Poster.
- LÉVESQUE, É.; LAPORTE, S. T.; VANIER, S.; CHARETTE, A. B.
- On-Demand Diattractor Diazo Reagents : In-Flow Generation and Purification
- Pacifichem, Honolulu, HI, 15-20 déc. 2015; Oral.
- MORIN, É.; BÉDARD, A.-C.; COLINS, S. K.
- In-Line Extraction and Purification of PEG Co-Solvents during Macrocyclizations Employing a "Phase Separation" Strategy
- 26th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), UQAM, Montréal, QC, 6-8 nov. 2015; Poster.
- PARISIEN COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Progress Toward New Photochemical Systems Using Fe-Based Sensitizers and O2
- 26th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), UQAM, Montréal, QC, 6-8 nov. 2015; Poster.
- SANTANDREA, J.; BÉDARD, A.-C.; MINOZZI, C.; COLLINS. S. K.
- Advances in Thermal and Photochemical Cu(I)-Catalyzed Macrocyclic Sonogashira-Type Cross-Couplings
- 26th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), UQAM, Montréal, QC, 6-8 nov. 2015; Poster.
- CARON, A.; HERNANDEZ PEREZ, A. C.; COLLINS, S. K.
- A U.V. Light Mediated Synthesis of Carbazoles via Flow Chemistry
- 26th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), UQAM, Montréal, QC, 6-8 nov. 2015; Poster.
- DE LÉSÉLEUC, M.; GODIN, E.; PARISIEN-COLLETTE, S.; COLLINS, S. K.
- Progress Toward the Total Synthesis of Ivorenolide A
- 26th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), UQAM, Montréal, QC,6-8 nov. 2015; Poster.
- VANIER, S.; LÉVESQUE, E.; CHARETTE, A. B.
- Génération et purification de composés diazo diattracteurs en flux continu
- 27e colloque annuel des étudiantes et étudiants en chimie de l'Université de Sherbrooke, Sherbrooke, QC, 16 oct. 2015; Poster.
- CHARETTE, A. B.
- New Methods in Organic Synthesis
- Université de Genève, Genève, Suisse, 1 oct. 2015; Conférence sur invitation.
- MASSON, J. F.
- Microdialysis SPR: Sensing in Whole Blood
- The Great Scientific Exchange (SciX), Providence, RI, 27 sept. - 2 oct. 2015; Conférence sur invitation.
- COLLINS, S. K.
- New Strategies for Macrocyclization
- University of California at Irvine, Irvine, CA, 17 sept. 2015; Conférence sur invitation.
- COLLINS, S. K.
- Catalysis and Continuous Flow Strategies in Macrocyclization
- Flow Chemistry Congress, San Diego, CA, 15-16 sept. 2015; Conférence sur invitation.
- CARON, A.; COLLINS, S. K.
- U.V. Light-Mediated Synthesis of Carbazoles via Flow Chemistry
- Flow Chemistry Congress, San Diego, CA, 15-16 sept. 2015; Poster.
- KAIROUZ, V.; COLLINS, S.; Charette, A. B.
- NSERC CREATE Program in Continuous Flow Science
- Flow Chemistry Congress, San Diego, CA, 15-16 sept. 2015; Poster.
- MONGEON, S.; MACCHI, A.
- Micro-Reactor Design for Fast Liquid-Liquid Reactions
- Flow Chemistry Congress, San Diego, CA, 15-16 sept. 2015; Poster.
- PLOUFFE, P.; MACCHI, A.
- Reverse Pulsatile Flow Reactor Applied to an Immiscible Liquid-Liquid System
- Flow Chemistry Congress, San Diego, CA, 15-16 sept. 2015; Poster.
- LÉVESQUE, É.; LAPORTE, S. T.; VANIER, S.; CHARETTE, A. B.
- On-Demand Diattractor Diazo Reagents : In-Flow Generation and Purification
- 250th ACS National Meeting, Boston, MA, 16-20 août 2015; Poster.
- MASSON, J. F.
- Nanomaterials and Plasmonic Devices for Small Molecule Monitoring
- 48th IUPAC World Congress, Busan, Corée, 7-13 août 2015; Conférence sur invitation.
- CHARETTE, A. B.
- Nouvelles méthodes en synthèse organique
- Janssen R&D, Val de Reuil, France, 1 juil. 2015; Conférence sur invitation.
- CHARETTE, A. B.
- Fundamentals and Applications of Flow Chemistry
- INSA-Rouen, Rouen, France, 30 juin 2015; Conférence sur invitation.
- CHARETTE, A. B.
- New Methods in Organic Synthesis
- Oril Industrie (Servier), Bolbec, France, 29 juin 2015; Conférence sur invitation.
- COLLINS, S. K.
- Improved Macrocyclization Protocols Using Continuous Flow Methods
- Physical Organic Chemistry, Gordon Research Conference Holderness, NH, 23 juin 2015; Conférence sur invitation.
- BÉDARD, A.-C.; SANTANDREA, J.; COLLINS, S. K.
- Advanced Strategies for Efficient Macrocyclization in Continuous Flow
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- DE LÉSÉLEUC, M.; GODIN, E.; PARISIEN- COLLETTE, S.; COLLINS, S. K.
- Progress toward the Total Synthesis of Ivorenolide A
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- SANTANDREA, J., BÉDARD, A.-C.; COLLINS, S. K.
- Advances in Thermal and Photochemical Cu(I)-catalyzed Macrocyclic Sonogashira-Type Cross-Couplings
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- PARISIEN-COLLETTE, HERNANDEZ-PEREZ, A. C.; S.; COLLINS, S. K.
- Photoredox of Heterocycles in Continuous Flow
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- CARON, A.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- U. V. Light Mediated Carbazoles Synthesis by a Photocyclodehydrogenation Reaction in Flow
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- LÉVESQUE, É.; LAPORTE, S. T.; CHARETTE, A. B.
- Generation and Purification of Reactive Diazo Solutions in Continuous Flow
- 98th Canadian Chemistry Conference and Exhibition, Ottawa, ON, 13-17 juin 2015; Oral.
- MA, Z.; TREVISANUT, C. NEAOGE, C.; BOFFITO, D. C.; JAZAYERI, S. M.; JAGPAL, C.; PATIENCE, G. S.
- A Micro-Refinery Unit for the Gas-to-Liquid Transformation of Associated Natural Gas
- EIC's 4th Climate Change Technology Conference, Montréal, QC, 25-27 mai 2015; Oral.
- CHARETTE, A. B.
- Building Up a World-Class Flow Infrastructure at Université de Montréal: Applications to Cyclopropane Chemistry
- G3 de la francophonie, Univ. Libre de Bruxelles, Brussels, Belgium, 22 mai 2015; Conférence sur invitation.
- CHARETTE, A. B.
- Synthèse et applications de nouveaux dérivés hétérocycles
- Univ. d'Orléans, Orléans, France, 20 mai 2015; Conférence sur invitation.
- COLLINS, S. K.
- Continuous Flow Synthesis in Green Chemistry and Catalysis
- 6e conférence annuelle du CCVC, Québec, QC, 12 mai 2015; Conférence sur invitation.
- CHARETTE, A. B.
- Organic Synthesis in the 21st Century: Challenges and Opportunities
- Univ. de Rouen, Rouen, France, 3 avr. 2015; Conférence sur invitation.
- AUBÉ, A.; CHARBONNEAU, D.; PELLETIER, J. N.; MASSON, J.-F.
- Anti-Asparaginase SPR Sensing to Detect Allergic Reactions in Leukemia Treatment
- 2nd International Conference on Label-Free Technologies, Cambridge, MA, 12-14 mars 2015; Poster.
- MASSON, J.-F.
- A Four-Channel Portable SPR Instrument to Quantify Therapeutic Drugs and Other Small Molecules
- Pittcon Conference and Expo, New Orleans, LA, 8-12 mars 2015; Conférence sur invitation.
- COLLINS, S. K.
- Photochemical Continuous Flow Strategies
- 5th International Conference of the Flow Chemistry Society, Berlin, Allemagne, 17-18 févr. 2015; Conférence sur invitation.
- COLLINS, S. K.
- Continuous Flow Chemistry
- University of Cambridge, Cambridge, Royaume-Uni, 12 févr. 2015; Conférence sur invitation.
- MASSON, J. F.; BREAULT-TURCOT, J.; AUBÉ, A.; FOREST, S.; CHAURAND, P.
- SPR-MS: From Identifying Adsorbed Molecules to Image Tissues Photonics SPIE Photonics
- West Conference, San Francisco, CA, 7-12 févr. 2015; Conférence sur invitation.
- COLLINS, S. K.
- Towards a Green Macrocyclization Protocol: Novel Catalysis, Concentration Control and Continuous Flow Strategies
- University of Windsor, Windsor, ON, 29 janv. 2015; Conférence sur invitation.
- COLLINS, S. K.
- Towards a Green Macrocyclization Protocol: Novel Catalysis, Concentration Control and Continuous Flow Strategies
- Wayne State University, Detroit, MI, 28 janv. 2015; Conférence sur invitation.
- CHARETTE, A. B.
- New Methods for the Formation of Useful Organic Scaffolds: From Functionalized Cyclopropanes to Heterocycle Derivatives
- Univ. of Toronto, Toronto, ON, 4 déc. 2014; Conférence sur invitation.
- PARISIEN-COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Photoredox Catalysis Employing Fe-Based Sensitizers: A Visible-Light-Mediated Synthesis of Carbazoles
- 17th Annual Chemistry and Biochemistry Graduate Research Conference, Concordia University, Montréal, QC, 28 nov. 2014; Oral.
- CARON, A.; COLLINS, S. K.
- UV Light-Mediated Synthesis of Carbazoles Via Flow Chemistry
- 17th Annual Chemistry and Biochemistry Graduate Research Conference, Concordia University, Montréal, QC, 28 nov. 2014; Oral.
- BÉDARD, A.-C.; COLLINS, S. K.
- Progress Toward Efficient Macrocyclization Reaction: Exploiting Phase Separation to Control Dilution Effects
- Commonwealth Science Conference, Bangalore, India, 25-28 nov. 2014; Poster.
- CHARETTE, A. B.
- Short Course on New Methods in Organic Synthesis
- OmegaChem, Quebec City, QC, 10 et 24 nov. 2014; Conférences sur invitation.
- PARISIEN-COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Photoredox Catalysis Employing Fe-Based Sensitizers: A Visible-Light-Mediated Synthesis of Carbazoles
- 25th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), Ryerson University, Toronto, ON, 7-9 nov. 2014; Poster.
- CARON, A.; HERNANDEZ PEREZ, A. C.; COLLINS, S. K.
- UV Light Mediated Synthesis of Carbazole: Application of a UV Reactor Toward the Construction of a Carprofen Analog
- 25th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), Ryerson University, Toronto, ON, 7-9 nov. 2014; Oral.
- BÉDARD, A.-C.; COLLINS, S. K.
- Advanced Strategies for Efficient Macrocyclic Cu(I)-Catalyzed Cycloaddition of Azides
- 25th Quebec and Ontario Mini-Symposium on Biological and Organic Chemistry (QOMSBOC), Ryerson University, Toronto, ON, 7-9 nov. 2014; Poster.
- LAPORTE, S.; LÉVESQUE, E.; CHARETTE, A. B.
- Synthèse et purification en écoulement continu de solutions réactives d'aryldiazométhanes via une pyrolyse en conditions douces
- 26e colloque annuel des étudiantes et étudiants en chimie de l'Université de Sherbrooke, Sherbrooke, QC, 17 oct. 2014; Oral.
- FOREST, S.; MASSON, J.-F.
- SPRi-MALDI-MS Method for Quantitative and Qualitative Imaging of Proteins in Biological Tissues
- The Great Scientific Exchange (SciX), Reno, NV, 28 sept.-2 oct. 2014; Poster.
- MASSON, J.-F.
- A Four-Channel SPR Fluorescence Instrument
- The Great Scientific Exchange (SciX), Reno, NV, 28 sept.-2 oct. 2014; Conférence sur invitation.
- AUBÉ, A.; CHARBONNEAU, D.; PELLETIER, J. N.; MASSON, J.-F.
- Anti-Asparaginase Sensing to Detect Allergic Reactions in Leukemia Treatment Production of Nanoelectrodes
- 10th Student Symposium of the Centre for Self-Assembled Chemical Structures (CSACS), Université de Montréal, Montréal, QC, 8 sept. 2014; Poster.
- PARISIEN-COLLETTE, S.; HERNANDEZ-PEREZ, A. C.; COLLINS, S. K.
- Visible-Light-Mediated Synthesis of Carbazoles
- 10th Student Symposium of the Centre for Self-Assembled Chemical Structures (CSACS), Université de Montréal, Montréal, QC, 8 sept. 2014; Poster.
- BÉDARD, A.-C.; VLASSOVA, A.; HERNANDEZ-PEREZ, A. C.; BESSETTE, A.; HANAN, G. S.; HEUFT, M. A.; COLLINS, S. K.
- Synthesis, Crystal Structure and Photophysical Properties of a Novel Pyrene/Helicene Hybrid
- 10th Student Symposium of the Centre for Self-Assembled Chemical Structures (CSACS), Université de Montréal, Montréal, QC, 8 sept. 2014; Poster.
- PLOUFFE, P.; MACCHI, A.
- Liquid-Liquid Mass Transfert Dynamics in Various Micro-Reactor.
- 13th International Conference on Microreaction Technology, Budapest, Hongrie, 23-25 juin 2014; Oral.
- AUBÉ, A.; CHARBONNEAU, D.; PELLETIER, J. N.; MASSON, J.-F.
- Anti-Asparaginase SPR Sensing to Detect Allergic Reactions in Leukemia Treatment
- Gordon Research Conference on Bioanalytical Sensors, Salve Regina University, Newport, RI, 22-27 juin 2014; Poster.
- POIRIER-RICHARD, H.-P.; MASSON, J.-F.
- Surface Enhanced Fluorescence on Gold Microhole Arrays, Combination of SPR and Fluorescence Applied to Biodetection
- Gordon Research Conference on Bioanalytical Sensors, Salve Regina University, Newport, RI, 22-27 juin 2014; Poster.
- CHARETTE, A. B.
- Enantio- and Diastereoselective Cyclopropanation Reactions to Access Functionalized 1,2,3-Substituted Cyclopropanes
- 97th Canadian Chemistry Conference and Exhibition, Vancouver, BC, 1-5 juin 2014; Conférence sur invitation.
- FOREST, S.; MASSON, J.-F.
- SPRi-MALDI-MS Method for Quantitative and Qualitative Imaging of Proteins in Biological Tissues
- 16th Photonics North Conference, Montréal, QC, 28-30 mai 2014; Poster.
- HEILEMAN, K.; DAOUD, J.; TABRIZIAN, M.
- Development of a Dielectric Spectroscopy Sensing Platform for Rapid and Real Time Evaluation of Pancreatic Islet Functionality
- 24th Anniversary-World Congress on Biosensors, Melbourne, Australie, 27-30 mai 2014; Oral.
- COLLINS, S. K.
- Towards a Green Macrocyclization Protocol: Development of Novel Strategies and Catalysis
- University of Michigan, Ann Arbor, MI, 18 mars 2014; Conférence sur invitation.
- CHARETTE, A. B.
- Nouvelles méthodes de synthèse de dérivés cyclopropaniques fonctionnalisés et d'hétérocycles
- Univ. du Québec à Montréal, Montréal, QC, 13 mars 2014; Conférence sur invitataion.
- POIRIER-RICHARD, H.-P.; BREAULT-TURCOT, J.; MASSON, J.-F.
- Metal Enhanced Fluorescence on Gold Microhole Arrays Towards a Dual Detection of a PSA Immunoassay
- Pittcon Conference & Expo, Chicago, IL, 2-6 mars 2014; Oral.
- AUBÉ, A.; BREAULT-TURCOT, J.; MASSON, J.-F.
- Highly Efficient Peptide Self-Assembled Monolayers to Reduce Non Specific Adsorption of Crude Cell Lysate on SPR Biosensors
- Pittcon Conference & Expo, Chicago, IL, 2-6 mars 2014; Oral.
- CHARETTE, A. B.
- Part 1: Synthesis and Applications of Halocyclopropanes. Part 2: Heterocycles Synthesis Using the Electrophilic Amide Activation
- Pfizer, Groton, CT, 30 janv. 2014; Conférence sur invitation.
- GODIN, É.
- Application de la stratégie de séparation de phase pour la synthèse de macrocycles complexes
- Université de Montréal, Montréal, QC, 24 mars 2017; Oral.
- SAYES, M.
- Utilisation du diphénylsilane pour la formation de liaisons amide : application à la synthèse peptidique
- Université de Montréal, Montréal, QC, 10 mars 2017; Oral.
- MORIN, É.
- Synthèse de la néomarchantine A
- Université de Montréal, Montréal, QC, 24 févr. 2017; Oral.
- DIERCXSENS, N.
- Trifluorométhylation de molécules organiques : vers la synthèse de cyclopropanes trifluorométhylés
- Université de Montréal, Montréal, QC, 25 nov. 2016; Oral.
- PARISIEN-COLLETTE, S.
- Développement de nouvelles méthodologies photochimiques utilisant la chimie en débit continu pour la synthèse de carbazoles
- Université de Montréal, Montréal, QC, 18 nov. 2016; Oral.
- CARON, A.
- Synthèse photochimique de carbazoles et azahelicènes en utilisant la chimie en débit continu
- Université de Montréal, Montréal, QC, 18 mars 2016; Oral.
- SANTANDREA, J.
- Improved Catalysis for Macrocyclization Reactions
- Université de Montréal, Montréal, QC, 31 août 2015; Oral.
- POUPART, J.
- Synthèse et analyse confirmationnelle de motifs N-amino indazol-2-ones substitués en position 5
- Université de Montréal, Montréal, QC, 13 nov. 2015; Oral.
- CRIFAR, C.
- Synthèse d'hétérocycles azotés par réaction d'addition en cascade catalysée au cuivre
- Université de Montréal, Montréal, QC, 13 mai 2015; Oral.
- AUDUBERT, C.
- Développement en flux continu de méthylènation à partir de composés diazos
- Université de Montréal, Montréal, QC, 7 mai 2015; Oral.
- TAILLEMAUD, S.
- Synthèse de monohalocyclopropanes et études de carbénoïdes de zinc
- Université de Montréal, Montréal, QC, 2017; thèse de doctorat.
- Lire la thèse
- AUBÉ, A.
- Développement de chimie de surface pour la réduction de l'adsorption non spécifique de lysat cellulaire et application clinique de biocapteurs SPR
- Université de Montréal, Montréal, QC, 2017; thèse de doctorat.
- MIELKE, E.
- Study on the Transport Phenomena in Complex Micro-Reactors
- Université d'Ottawa, Ottawa, QC, 2017; mémoire de maîtrise appliquée.
- Lire la thèse
- LÉVESQUE, É.
- Techniques de catalyse et de flux continu pour faciliter la fermeture de molécules cycliques tendues
- Université de Montréal, Montréal, QC, 2016; thèse de doctorat.
- PIRAS, H.
- Synthèse de sulfilimines et de sulfoximines catalysée par les métaux de transition
- Université de Montréal, Montréal, QC, 2016; thèse de doctorat.
- Lire la thèse
- TIEN SING YOUNG, R. V.
- Design and Fabrication of a Simple, Cost-Effective, Passive, Continuous-Flow Microfluidic Device for One-Step Synthesis of Bioactive Molecule Loaded Liposomes
- Université McGIll, Montréal, QC, 2015; thèse de doctorat.
- Lire la thèse
- FOUDEH, A.
- Development of a Novel Biosensor for Rapid and Specific Detection of Viable Legionella Bacteria for On-site Applications
- Université McGill, Montréal, QC, 2015; thèse de doctorat.
- Lire la thèse
- RASODA, R.
- A Platform for Controlled Myelinatin: An Investigation Into Pattered Oligodendrocyte Growth
- Université McGill, Montréal, QC, 2015; mémoire de maîtrise.
- Lire le mémoire
- PLOUFFE, P.
- Micro-Reactor Design for Fast Liquid-Liquid Reactions
- Université d'Ottawa, Ottawa, ON, 2015; thèse de doctorat.
- Lire la thèse
- FOREST, S.
- Développement d'une méthode SPRi-MALDI-IMS pour la quantification et l'identification des protéines dans des empreintes de tissus biologiques
- Université de Montréal, Montréal, QC, 2015; mémoire de maîtrise.
- Lire le mémoire
- GAREAU, D.
- Design rationnel de nanothermomètres programmables à base d'ADN
- Université de Montréal, Montréal, QC, 2015; mémoire de maîtrise.
- Lire le mémoire
- BEAUDOIN, D.
- Préparation de réseaux organiques covalents monocristallins par polymérisation de composés polynitroso aromatiques
- Université de Montréal, Montréal, QC, 2015; thèse de doctorat.
- Lire la thèse
- BÉDARD, A.-C.
- Development of a Phase Separation Strategy in Macrocyclization Reactions
- Université de Montréal, Montréal, QC, 2015; thèse de doctorat.
- Lire la thèse
- HERNANDEZ PEREZ, A. C.
- Réaction de photocyclodéshydrogénation par catalyse photorédox
- Université de Montréal, Montréal, QC, 2015; thèse de doctorat.
- Lire la thèse
- CYR, P.
- Hydroxylation d'halogénures d'aryle utilisant la chimie en flux continu et développement d'une nouvelle méthodologie de synthèse de 3-aminoindazoles
- Université de Montréal, Montréal, QC, 2014; mémoire de maîtrise.
- Lire le mémoire

